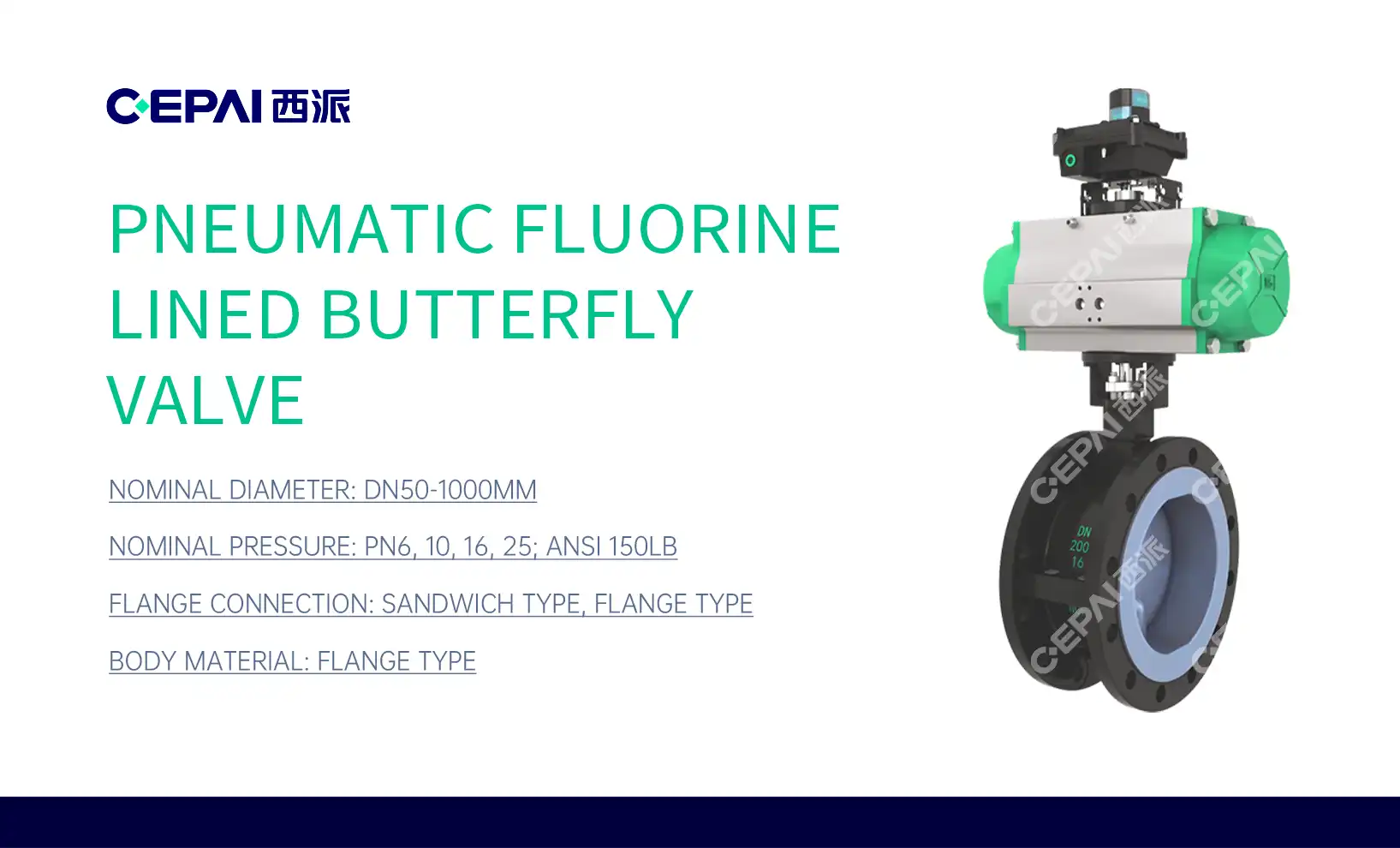
The Advantages of PTFE-Lined Valves in Pharmaceutical Manufacturing
Chemical Resistance and Inertness
PTFE-lined valves boast remarkable chemical resistance, making them suitable for handling a wide array of aggressive substances commonly used in pharmaceutical production. The inert nature of PTFE ensures that it does not react with or contaminate the fluids passing through the valve, preserving the purity of pharmaceutical products. This characteristic is crucial in maintaining the efficacy and safety of medications, as even minute contaminations can have significant consequences.
Contamination Prevention and Easy Cleaning
The non-stick surface of PTFE-lined butterfly valves prevents product buildup and reduces the risk of cross-contamination between batches. This property is particularly valuable in pharmaceutical manufacturing, where strict hygiene standards must be maintained. The smooth surface of PTFE facilitates easy cleaning and sterilization, allowing for quick turnaround times between production runs and minimizing the risk of bacterial growth or product residue.
Temperature and Pressure Tolerance
PTFE-lined valves exhibit excellent performance across a wide range of temperatures and pressures. This versatility makes them suitable for various pharmaceutical processes, from low-temperature storage to high-temperature sterilization. The ability to withstand extreme conditions without compromising their integrity or functionality ensures consistent performance and reliability in critical pharmaceutical applications.
Enhancing Process Efficiency with PTFE-Lined Butterfly Valves
Precise Flow Control
PTFE-lined butterfly valves offer exceptional flow control capabilities, allowing for accurate regulation of fluid flow in pharmaceutical processes. The smooth operation and tight sealing properties of these valves enable precise dosing and mixing of ingredients, crucial for maintaining product consistency and quality. The ability to fine-tune flow rates contributes to improved process efficiency and reduced waste, ultimately leading to cost savings for pharmaceutical manufacturers.
Reduced Maintenance and Downtime
The durability and resistance to wear of PTFE-lined butterfly valves translate to reduced maintenance requirements and extended service life. This characteristic is particularly beneficial in pharmaceutical manufacturing, where unplanned downtime can result in significant production losses and potential product quality issues. By minimizing the need for frequent valve replacements or repairs, PTFE-lined butterfly valves help maintain continuous operations and enhance overall plant efficiency.

Compact Design and Space Efficiency
PTFE-lined butterfly valves feature a compact design that makes them ideal for installations with space constraints. Their slim profile allows for easy integration into existing piping systems without requiring extensive modifications. This space efficiency is particularly advantageous in pharmaceutical facilities, where equipment density is often high, and every inch of floor space is valuable. The compact nature of these valves also contributes to reduced weight and easier handling during installation and maintenance procedures.
Regulatory Compliance and Safety Considerations
Meeting FDA and GMP Standards
PTFE-lined valves play a crucial role in helping pharmaceutical manufacturers comply with stringent regulatory requirements, including FDA regulations and Good Manufacturing Practice (GMP) standards. The inert nature of PTFE and its resistance to bacterial growth align with the strict hygiene and contamination control mandates in the pharmaceutical industry. By incorporating PTFE-lined valves into their processes, companies can demonstrate their commitment to maintaining the highest standards of product quality and safety, facilitating regulatory approvals and inspections.
Traceability and Documentation
Many PTFE-lined butterfly valve manufacturers provide comprehensive documentation and traceability for their products, including material certifications, testing reports, and quality assurance records. This documentation is invaluable for pharmaceutical companies in maintaining their quality management systems and demonstrating compliance with regulatory requirements. The ability to trace the origin and performance history of each valve component contributes to overall product quality assurance and facilitates swift resolution of any potential issues that may arise during production or post-market surveillance.
Worker Safety and Environmental Considerations
The use of PTFE-lined valves in pharmaceutical applications also contributes to improved worker safety and environmental protection. The chemical resistance of PTFE reduces the risk of leaks or spills that could expose workers to hazardous substances. Additionally, the longevity and durability of these valves result in less frequent replacements, reducing waste generation and the environmental impact associated with valve disposal. These factors align with the growing emphasis on sustainability and responsible manufacturing practices in the pharmaceutical industry.
Conclusion
PTFE-lined valves, particularly PTFE-lined butterfly valves, offer numerous advantages for pharmaceutical applications. Their chemical resistance, contamination prevention capabilities, and efficiency-enhancing features make them an excellent choice for maintaining product integrity and optimizing manufacturing processes. By investing in high-quality PTFE-lined valves, pharmaceutical companies can ensure compliance with regulatory standards, improve operational efficiency, and ultimately deliver safer, higher-quality products to patients worldwide.
FAQs
How long do PTFE-lined valves typically last in pharmaceutical applications?
The lifespan of PTFE-lined valves can vary depending on the specific application and operating conditions. However, with proper maintenance, they can often last for several years, providing excellent value for pharmaceutical manufacturers.
Are PTFE-lined valves suitable for high-purity water systems in pharmaceutical facilities?
Yes, PTFE-lined valves are well-suited for high-purity water systems due to their inert nature and resistance to bacterial growth, making them ideal for maintaining water quality in pharmaceutical production.
Can PTFE-lined valves handle abrasive slurries or powders in pharma processes?
While PTFE-lined valves offer excellent chemical resistance, their suitability for highly abrasive materials may depend on the specific valve design and application. Consulting with a valve specialist is recommended for such scenarios.
Choose CEPAI for Your PTFE-Lined Valve Needs
CEPAI Group Co., Ltd. specializes in manufacturing high-quality PTFE-lined valves for the pharmaceutical industry. Our advanced manufacturing processes and strict quality control ensure that our valves meet the highest standards of performance and reliability. As a leading valve manufacturer and factory, we offer customized solutions to meet your specific pharmaceutical application needs. Contact us at cepai@cepai.com to learn how our PTFE-lined butterfly valves can enhance your production processes.

References
Johnson, A. (2022). Advanced Materials in Pharmaceutical Valve Applications. Journal of Pharmaceutical Engineering, 45(3), 178-192.
Smith, B., & Brown, C. (2021). PTFE-Lined Valves: A Comprehensive Guide for the Pharmaceutical Industry. New York: PharmaTech Press.
Zhang, L., et al. (2023). Comparative Analysis of Valve Materials in High-Purity Pharmaceutical Processes. International Journal of Pharmaceutical Manufacturing, 12(2), 89-104.
Thompson, R. (2020). Regulatory Compliance in Pharmaceutical Valve Selection. Pharmaceutical Technology, 44(9), 56-62.
Davis, M., & Wilson, K. (2022). Efficiency Gains Through Advanced Valve Technologies in Pharma Manufacturing. BioPharm International, 35(4), 22-28.
Lee, S., et al. (2021). Long-term Performance Evaluation of PTFE-Lined Valves in Aggressive Pharmaceutical Environments. Journal of Materials in Pharmaceutical Processing, 18(3), 305-319.

_1746598525968.webp)