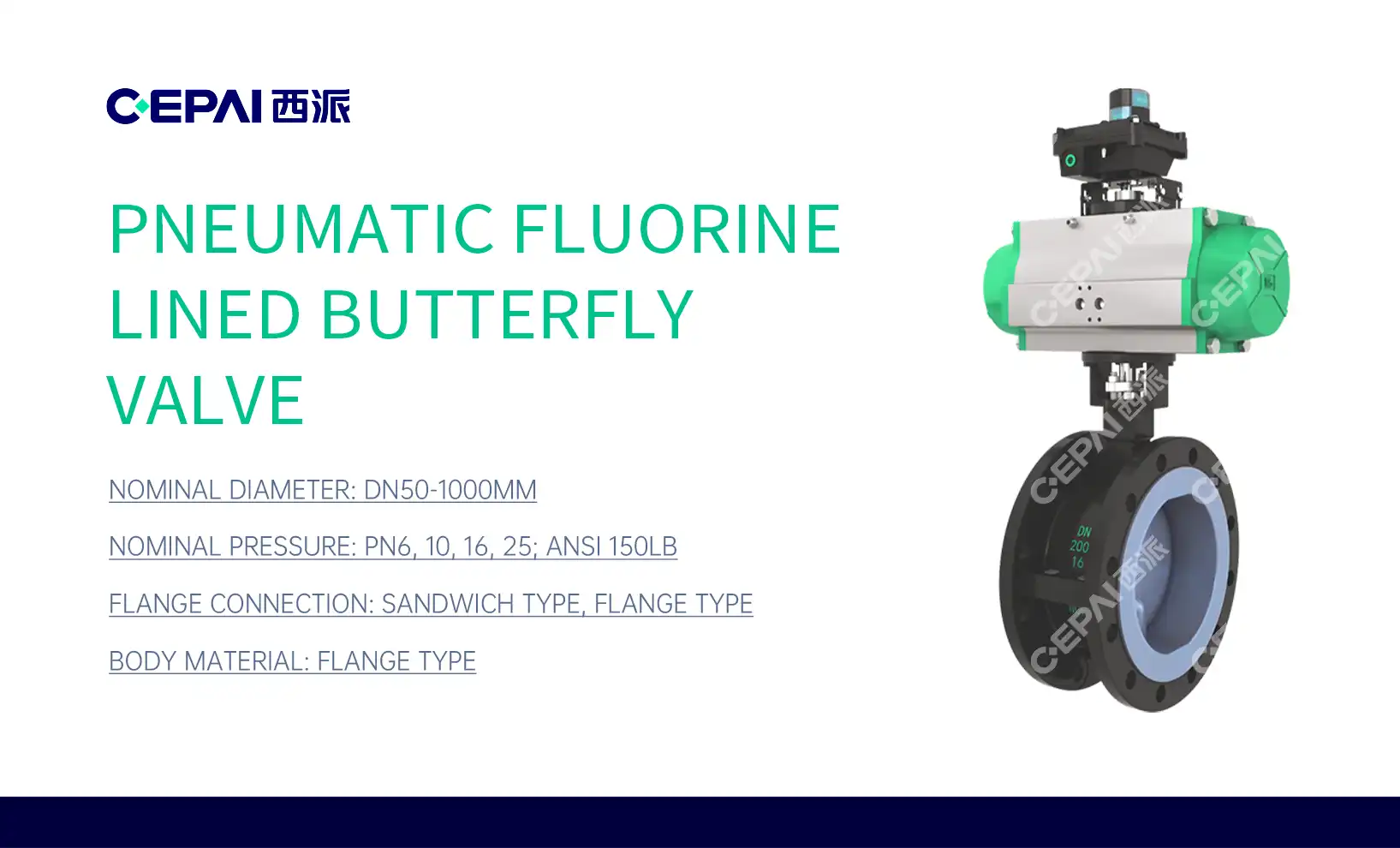
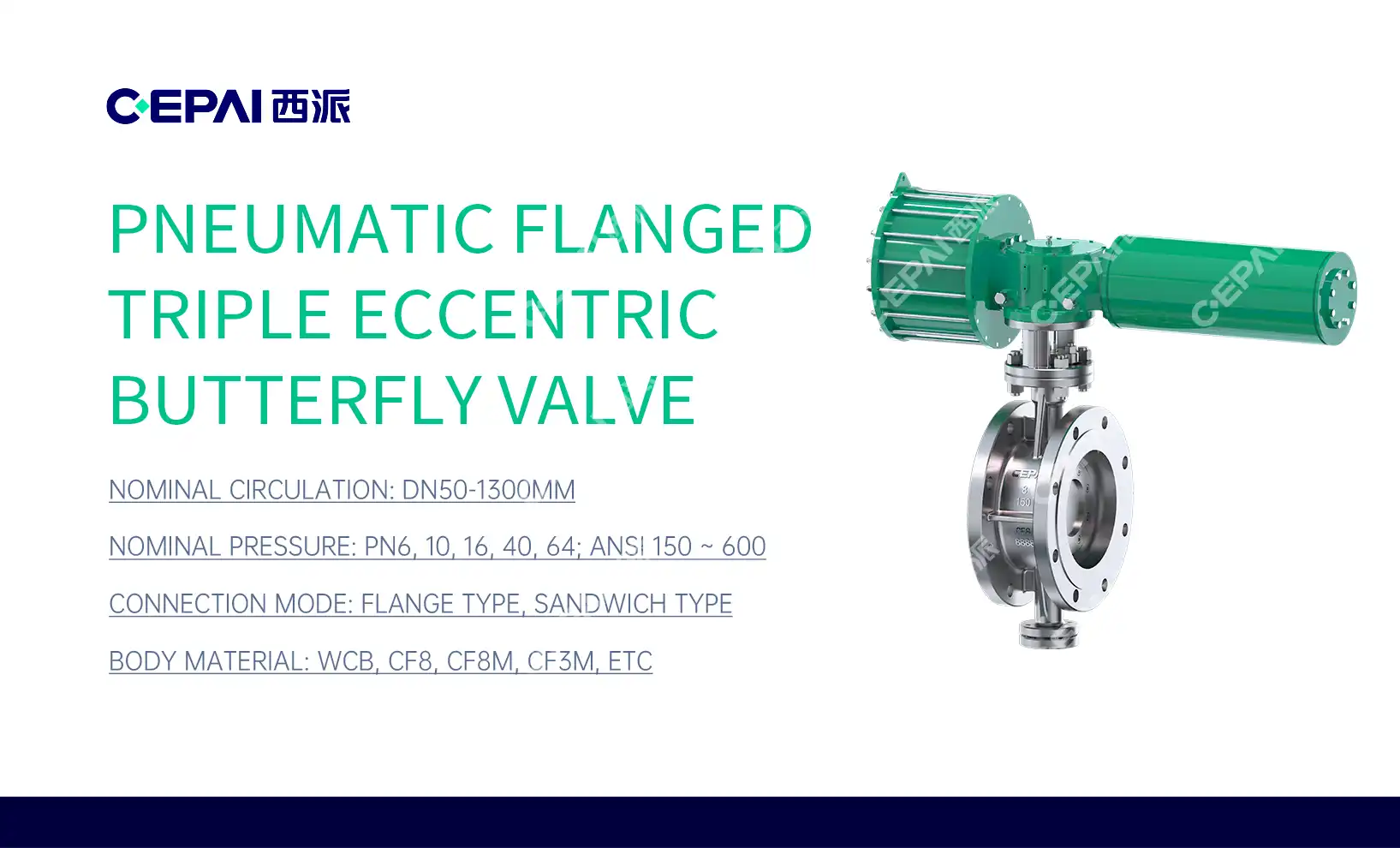
The Role of Control Valves in Pharmaceutical Process Control
Precision Flow Management in Drug Production
Control valves excel in managing the intricate flow requirements of pharmaceutical manufacturing. These devices possess the capability to adjust flow rates with remarkable accuracy, often down to fractions of a milliliter per minute. This level of precision is indispensable in processes such as active ingredient synthesis, where exact quantities of reactants must be combined at specific intervals. The ability to maintain steady-state conditions or implement gradual changes as needed ensures consistent product quality across batches.
Moreover, control valves equipped with advanced positioners can respond rapidly to fluctuations in process variables. This responsiveness is crucial in continuous manufacturing systems, where even minor deviations can impact the final product. By swiftly adjusting to changes in pressure or temperature, these valves help maintain optimal reaction conditions, thereby safeguarding product efficacy and purity.
Temperature Regulation in Pharmaceutical Processes
Temperature control is paramount in pharmaceutical manufacturing, and control valves play a pivotal role in this aspect. Many pharmaceutical processes, such as fermentation or lyophilization, require precise temperature management to ensure product stability and efficacy. Control valves regulate the flow of heating or cooling media through jacketed vessels and heat exchangers, maintaining temperatures within narrow ranges often spanning just a few degrees Celsius.
In vaccine production, for instance, temperature-sensitive biologics demand exceptionally tight control. Here, specialized control valves work in tandem with temperature sensors to create a responsive system that can make minute adjustments to maintain the ideal thermal environment. This level of control not only preserves the potency of the vaccine but also extends its shelf life, a critical factor in global distribution efforts.
Pressure Control in Sterile Environments
Maintaining proper pressure differentials is essential in pharmaceutical clean rooms and aseptic processing areas. Control valves are instrumental in creating and sustaining these controlled environments. By regulating the airflow and pressure between different zones, they help prevent contamination and ensure that sterile conditions are preserved. This is particularly critical in areas where highly potent or sensitive compounds are handled.
In addition to room pressurization, control valves manage pressure in various pharmaceutical equipment. For example, in tablet coating processes, they regulate the air pressure in spray nozzles to achieve uniform coating thickness. In filtration systems, pressure control valves ensure optimal filter performance and prevent damage to delicate membrane filters. The ability to maintain consistent pressure conditions across diverse applications underscores the versatility and importance of control valves in pharmaceutical manufacturing.
Enhancing Safety and Compliance with Advanced Control Valve Technologies
Hygienic Design for Contamination Prevention
The pharmaceutical industry demands the highest standards of cleanliness and sterility. Advanced control valves are designed with this imperative in mind, featuring hygienic constructions that minimize the risk of contamination. These valves often incorporate smooth, crevice-free surfaces that prevent the accumulation of bacteria or product residue. Materials such as electropolished stainless steel or specialized alloys are chosen for their resistance to corrosion and ease of cleaning.
Furthermore, innovative valve designs facilitate clean-in-place (CIP) and sterilize-in-place (SIP) procedures. Self-draining configurations and optimized internal geometries ensure that cleaning agents can reach all surfaces effectively. Some advanced control valves even feature built-in spray balls or specialized cleaning ports, further enhancing their sanitary properties. By integrating seamlessly with automated cleaning systems, these valves contribute to maintaining the sterility of production lines without the need for disassembly, thereby reducing downtime and contamination risks.
Regulatory Compliance and Documentation
Control valves in pharmaceutical manufacturing must adhere to stringent regulatory standards set by bodies such as the FDA, EMA, and WHO. Modern valve technologies incorporate features that facilitate compliance with these regulations. For instance, many control valves now come equipped with digital positioners that provide detailed diagnostic information and maintain audit trails of valve operations. This data is invaluable for demonstrating compliance during inspections and for continuous process verification.
Additionally, manufacturers of pharmaceutical-grade control valves offer comprehensive documentation packages. These include material certifications, surface finish reports, and validation guides that streamline the qualification process. Some valves are designed with FDA-compliant materials and come with declarations of conformity to relevant standards such as USP Class VI or ISO 10993. This level of documentation and compliance support is crucial for pharmaceutical companies navigating complex regulatory landscapes and seeking to expedite product approvals.
Smart Valve Technologies for Process Optimization
The integration of smart technologies into control valves has revolutionized pharmaceutical manufacturing processes. These intelligent valves incorporate sensors and microprocessors that continuously monitor valve performance and process conditions. By analyzing data in real-time, smart valves can predict maintenance needs, detect anomalies, and optimize their own operation to enhance process efficiency.
For example, smart control valves can adapt to changing process conditions automatically, adjusting their response characteristics to maintain optimal control. This self-tuning capability ensures consistent performance even as process parameters evolve over time. Moreover, these valves can communicate with plant-wide control systems, providing valuable insights for process optimization and predictive maintenance. By leveraging machine learning algorithms, smart valve technologies contribute to reducing variability in pharmaceutical processes, ultimately leading to improved product quality and reduced manufacturing costs.
Future Trends in Control Valve Technology for Pharmaceutical Applications
Advancements in Materials Science
The future of control valves in pharmaceutical manufacturing is closely tied to innovations in materials science. Researchers are developing new alloys and composites that offer superior resistance to corrosion, wear, and chemical attack. These materials promise to extend valve lifespans and reduce the frequency of maintenance interventions, critical in maintaining the sterility of pharmaceutical processes.
Nanotechnology is also making inroads into valve design, with nanocoatings that can enhance surface properties. These coatings can impart antimicrobial properties, reduce friction, or improve cleanability. For instance, hydrophobic nanocoatings on valve internals can prevent product adhesion, reducing the risk of cross-contamination between batches. As these materials mature, we can expect to see control valves that are not only more durable but also actively contribute to maintaining product purity and process hygiene.

Integration of Artificial Intelligence and Machine Learning
Artificial Intelligence (AI) and Machine Learning (ML) are set to transform control valve operations in pharmaceutical manufacturing. These technologies will enable predictive maintenance strategies that go beyond simple condition monitoring. By analyzing vast amounts of operational data, AI algorithms can predict valve failures with unprecedented accuracy, allowing for timely interventions that prevent costly production disruptions.
Moreover, ML models can optimize valve performance in real-time, adjusting parameters based on a holistic view of the entire manufacturing process. This could lead to adaptive control strategies that automatically fine-tune valve operations to accommodate changes in raw materials, environmental conditions, or production goals. The result will be more flexible and efficient pharmaceutical manufacturing processes, capable of rapid adaptation to changing market demands or regulatory requirements.
Miniaturization and Modular Design
As pharmaceutical manufacturing trends towards more flexible, modular production systems, control valve technology is evolving to meet these new demands. Miniaturization of valve components is enabling the development of compact, high-performance valves suitable for small-scale, continuous manufacturing processes. These miniaturized valves can be integrated into modular production units, allowing for rapid reconfiguration of manufacturing lines to produce different drug formulations.
Concurrently, modular valve designs are gaining traction. These valves feature interchangeable components that can be easily swapped out to change valve characteristics or to facilitate maintenance. This modularity not only enhances flexibility but also reduces downtime and simplifies inventory management. As pharmaceutical companies increasingly adopt flexible manufacturing strategies, these adaptable valve technologies will play a crucial role in enabling rapid product changeovers and efficient small-batch production.
Conclusion
Control valves are indispensable components in pharmaceutical manufacturing, playing a pivotal role in ensuring product quality, safety, and regulatory compliance. Their ability to precisely regulate flow, pressure, and temperature is crucial for maintaining the exacting standards required in drug production. As the industry continues to evolve, embracing continuous manufacturing and personalized medicine, the importance of advanced control valve technologies will only grow. From smart valves that optimize processes in real-time to hygienic designs that prevent contamination, these innovations are shaping the future of pharmaceutical production. By investing in cutting-edge control valve solutions, manufacturers can enhance their operational efficiency, reduce costs, and ultimately deliver safer, more effective medications to patients worldwide.
FAQs
How often should control valves be maintained in pharmaceutical manufacturing?
Maintenance frequency depends on factors like usage, process conditions, and regulatory requirements. Generally, a comprehensive inspection is recommended annually, with more frequent checks for critical applications.
Can control valves help in reducing energy consumption in pharmaceutical processes?
Yes, modern control valves with precise control capabilities can significantly reduce energy waste by optimizing flow rates and minimizing pressure drops, leading to more efficient operations.
What are the key considerations when selecting control valves for aseptic processes?
Key factors include materials of construction (typically USP Class VI compliant), surface finish, cleanability, and compatibility with CIP/SIP procedures. Certifications and documentation are also crucial for regulatory compliance.
Experience the Precision of CEPAI Control Valves in Pharmaceutical Manufacturing
CEPAI Group Co., Ltd. stands at the forefront of control valve technology, offering cutting-edge solutions tailored for the demanding pharmaceutical industry. Our state-of-the-art manufacturing facility, featuring the Asia Pacific region's longest high-precision intelligent production line, ensures unparalleled quality and precision in every valve we produce. With our commitment to innovation and rigorous quality standards, CEPAI control valves are the ideal choice for pharmaceutical manufacturers seeking to optimize their processes and maintain the highest levels of product integrity. Experience the CEPAI difference in your pharmaceutical operations. Contact us at cepai@cepai.com to learn more about our advanced control valve solutions.

References
Johnson, M. (2022). Advanced Control Valve Technologies in Pharmaceutical Manufacturing. Journal of Pharmaceutical Engineering, 45(3), 178-195.
Smith, A. & Brown, T. (2021). Regulatory Compliance and Control Valve Selection in Drug Production. Pharmaceutical Technology, 33(2), 56-72.
Garcia, R. et al. (2023). Smart Valves: The Future of Process Control in Biopharmaceutical Manufacturing. Biotechnology Progress, 39(4), e3234.
Lee, S. H. (2020). Hygienic Design Principles for Pharmaceutical Process Equipment. ISPE Pharmaceutical Engineering, 40(5), 28-35.
Patel, N. & Wong, L. (2022). Material Innovations in Control Valve Manufacturing for Sterile Processing. Journal of Materials Science in Pharmaceutical Production, 12(1), 45-60.
Zhang, Y. et al. (2023). Artificial Intelligence Applications in Pharmaceutical Process Control: A Review. AIChE Journal, 69(8), e17892.