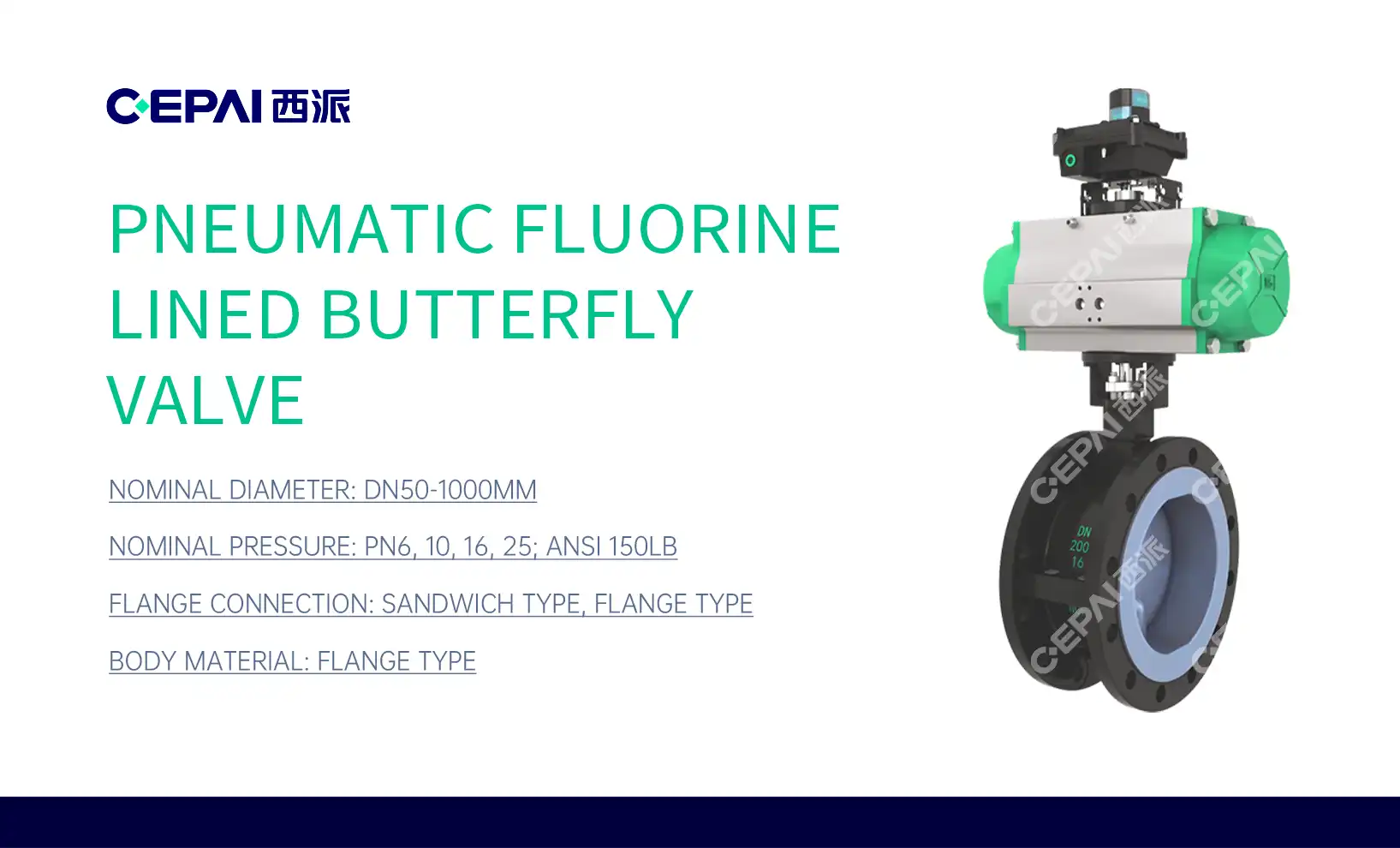
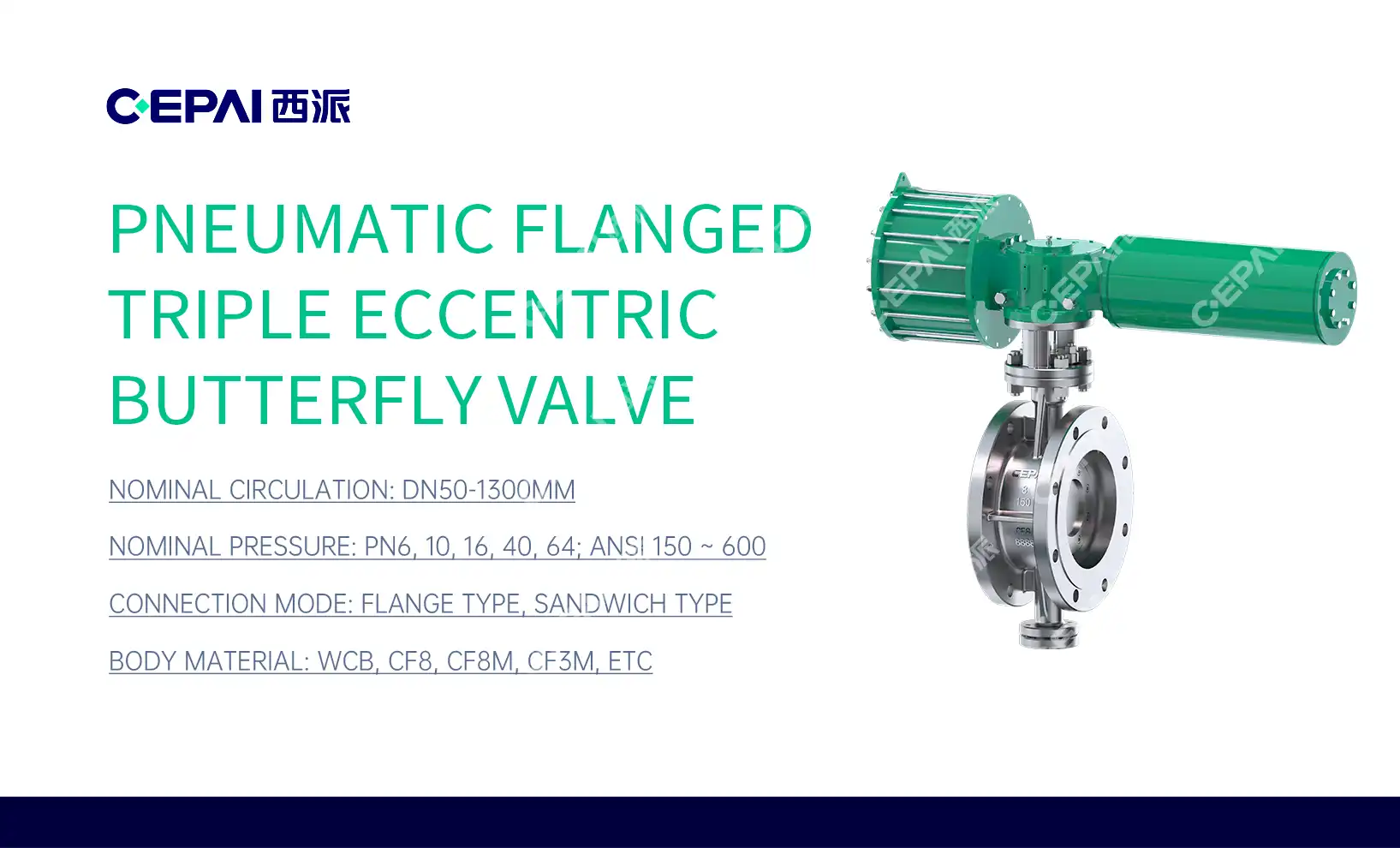
Regulatory Standards and Material Requirements for Pharmaceutical Valves
FDA Compliance and Material Specifications
The U.S. Food and Drug Administration (FDA) plays a pivotal role in setting standards for pharmaceutical valve materials. FDA 21 CFR Part 177 outlines requirements for materials that come into direct contact with food and drugs. This regulation ensures that valve components do not leach harmful substances into pharmaceutical products. Materials commonly used in pharmaceutical valves, such as stainless steel, PTFE, and certain elastomers, must meet these stringent requirements.
Additionally, the FDA's current Good Manufacturing Practices (cGMP) guidelines provide a framework for ensuring that pharmaceutical equipment, including valves, is designed and manufactured to maintain product quality and safety. These guidelines emphasize the importance of using materials that are inert, easily cleanable, and resistant to corrosion and degradation.
USP Class VI Testing for Biocompatibility
The United States Pharmacopeia (USP) Class VI is a crucial standard for materials used in pharmaceutical valves. This classification involves rigorous testing to ensure biocompatibility and the absence of toxic substances. Materials that pass USP Class VI testing are considered safe for use in pharmaceutical applications, as they demonstrate minimal biological reactivity.
USP Class VI testing includes in vivo implantation tests, systemic toxicity tests, and intracutaneous reactivity tests. These tests evaluate the material's potential to cause adverse reactions when in contact with living tissues or bodily fluids. For pharmaceutical valve manufacturers, obtaining USP Class VI certification for valve components is often a prerequisite for use in critical pharmaceutical processes.
ASME BPE Standards for Hygienic Design
The American Society of Mechanical Engineers (ASME) Bioprocessing Equipment (BPE) standard is a comprehensive guideline for the design and construction of equipment used in biopharmaceutical manufacturing. This standard is particularly relevant for pharmaceutical valve selection, as it addresses key aspects such as material selection, surface finish requirements, and hygienic design principles.
ASME BPE standards specify requirements for surface roughness, which is critical for preventing bacterial adhesion and ensuring effective cleaning. The standard also provides guidelines for valve design features that promote cleanability and reduce the risk of contamination. Compliance with ASME BPE standards ensures that pharmaceutical valves meet the highest standards of hygienic design and performance.

Performance and Design Considerations in Pharmaceutical Valve Selection
Cleanability and Sterilization Capabilities
In pharmaceutical manufacturing, the ability to thoroughly clean and sterilize valves is paramount. Valve designs that incorporate features such as smooth internal surfaces, minimal crevices, and self-draining configurations are essential. These design elements facilitate effective cleaning-in-place (CIP) and sterilization-in-place (SIP) processes, which are standard in pharmaceutical operations.
Valve manufacturers often provide documentation on their products' cleanability and sterilization compatibility. This information may include recommended cleaning procedures, maximum sterilization temperatures, and cycle life under sterilization conditions. When selecting pharmaceutical valves, it's crucial to consider these factors to ensure that the valves can withstand repeated cleaning and sterilization without compromising their integrity or performance.
Flow Characteristics and Control Precision
The flow characteristics of pharmaceutical valves are critical for maintaining precise control over process parameters. Factors such as Cv (flow coefficient), rangeability, and linearity of control must be carefully evaluated. These characteristics affect the valve's ability to accurately regulate flow rates and pressures, which is essential in pharmaceutical processes where precise dosing and mixing are required.
Advanced valve designs, such as those incorporating equal percentage or linear flow characteristics, can provide more precise control over a wide range of operating conditions. When selecting valves for pharmaceutical applications, it's important to consider the specific process requirements and choose valves that offer the appropriate level of control precision.
Material Compatibility and Corrosion Resistance
The compatibility of valve materials with process fluids is a critical consideration in pharmaceutical valve selection. Valves must be constructed from materials that are resistant to corrosion, degradation, and chemical attack by the wide range of substances used in pharmaceutical manufacturing. Common materials include high-grade stainless steels (such as 316L), specialty alloys, and engineered plastics.
Corrosion resistance is particularly important in pharmaceutical applications, as even minor corrosion can lead to product contamination or compromise the valve's integrity. Manufacturers often provide corrosion resistance data for their valve materials, which should be carefully reviewed in the context of the specific process chemicals and conditions. In some cases, specialized coatings or linings may be used to enhance corrosion resistance for particularly aggressive environments.
Quality Assurance and Documentation Requirements for Pharmaceutical Valves
ISO 13485 Quality Management System
ISO 13485 is an international standard that specifies requirements for a quality management system in the medical device industry, which includes pharmaceutical equipment. While not specifically focused on valves, this standard provides a framework for ensuring consistent quality in the design, manufacture, and distribution of medical devices and related services.
For pharmaceutical valve manufacturers, compliance with ISO 13485 demonstrates a commitment to quality and regulatory compliance. This standard emphasizes risk management, process validation, and continuous improvement – all critical aspects in the production of high-quality pharmaceutical valves. When selecting valves, pharmaceutical companies often look for suppliers who are ISO 13485 certified as an indicator of their quality management practices.
Documentation and Traceability Requirements
Comprehensive documentation is a crucial aspect of pharmaceutical valve selection and use. This includes material certificates, test reports, and validation documentation. Material certificates, such as EN 10204 3.1 certificates, provide detailed information on the chemical composition and mechanical properties of the valve materials. These documents are essential for ensuring material traceability and compliance with regulatory requirements.
Test reports and validation documentation are equally important. These may include pressure test reports, leakage test results, and performance validation data. For critical applications, pharmaceutical companies may require extensive validation documentation, including installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ) protocols.
Compliance with cGMP and Validation Protocols
Adherence to current Good Manufacturing Practices (cGMP) is mandatory in pharmaceutical manufacturing. When selecting valves, it's essential to consider how they fit into the overall cGMP compliance strategy. This includes aspects such as ease of maintenance, cleanability, and the ability to integrate with automated control systems.
Validation protocols for pharmaceutical valves typically include rigorous testing to ensure they meet performance specifications under actual operating conditions. This may involve cycle testing, performance testing under various process conditions, and long-term reliability assessments. Valve manufacturers should be able to provide guidance and support for these validation processes, including recommended validation protocols and historical performance data.
Conclusion
Selecting the right pharmaceutical valve requires careful consideration of multiple standards and factors. From material compatibility and regulatory compliance to performance characteristics and documentation requirements, each aspect plays a crucial role in ensuring the safety, efficacy, and reliability of pharmaceutical manufacturing processes. By adhering to standards such as FDA regulations, USP Class VI, ASME BPE, and ISO 13485, and considering factors like cleanability, flow control, and material compatibility, pharmaceutical manufacturers can make informed decisions that support their quality and compliance objectives. Proper valve selection is not just about meeting regulatory requirements; it's about ensuring the integrity of the manufacturing process and, ultimately, the safety of the end product.
Contact Us
For expert guidance on pharmaceutical valve selection and to explore high-quality valve solutions that meet the most stringent industry standards, contact CEPAI Group. Our team of specialists can help you navigate the complexities of valve selection, ensuring you choose the right products for your specific pharmaceutical manufacturing needs. Reach out to us at cepai@cepai.com to learn how our valve solutions can enhance your manufacturing processes and support your compliance efforts.


_1746598538016.webp)